31+ Which Property Is True For Metals Pics
31+ Which Property Is True For Metals Pics. Metals are used for various purposes, from making wires and sheets. Metals can be bent and. Which property is true for metals? Molecular orbitals overlap to produce bands. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. Most metals conduct electricity and are very dull to the look. It cannot be separated into simpler parts. Which is a characteristic of the electron sea model for metallic bonding? The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. An element is a substance made up of one kind of atom; The properties of metals can be grouped into certain categories. Most metals are toxic if eaten and are hard. But does this definition fit with the true properties of metals? Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss.
Properties of metals,nonmetals and metalloids by Dajah Powel
Red Cedar 4x4 - Landscaping Materials - Wickliffe .... Molecular orbitals overlap to produce bands. An element is a substance made up of one kind of atom; Which property is true for metals? Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. It cannot be separated into simpler parts. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. Metals can be bent and. The properties of metals can be grouped into certain categories. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. But does this definition fit with the true properties of metals? Metals are used for various purposes, from making wires and sheets. Most metals are toxic if eaten and are hard. Which is a characteristic of the electron sea model for metallic bonding? Most metals conduct electricity and are very dull to the look.
Nickel on the opther hand is also a metal but does not conduct a lot of electricy.
During the first four processes metal is subjected to large amounts of strain (deformation). Although there are many similarities between metals, хотя разные металлы имеют много общего, there are also differences that determine how suitable a metal is for a. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. The total collection of pieces of property that serve to store value is a person's. Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (gallium is liquid on hot conduction: They can conduct electricity and heat. They can be formed easily. Many metals are very reactive and several nonmetals are nonreactive. Some chemical elements are called metals. Discussing this chapter, a classmate says, since elements that form cations are metals and elements that form anions are nonmetals, elements that do not form ions are metalloids. identify this saying as true or false. Metals can be drawn into a wire. A nonmetal is simply an element that does not display the properties of a metal. For example,lead is soft and can be bent by hand, whileiron can only be worked by hammering at red heat. The study of the production and properties of metals is known as metallurgy. Silver and copper are the two best conductors of heat and electricity. Metals are a group of elements that share certain properties. It cannot be separated into simpler parts. They are the majority of elements in the periodic table. But does this definition fit with the true properties of metals? They have a high melting point. Physical properties of metals and alloys. The evolution of the payments system from barter to precious metals, then to fiat money, then to checks can best be understood as a consequence of the fact that. They also have high melting points and. Metals vary greatly in their properties. Metals are materials most widely used in industry because of their properties. Most metals are toxic if eaten and are hard. It is a property of many metals, the ability to reflect light. Which property is true for metals? It is not defined by what it is, but by what it is not. Metals usually have high melting points. The study of the production and properties of metals is known as metals vary greatly in their properties.
mechanical engineering - How does a graph with tensile ...
CBSE 8, Science, CBSE-Materials-metals and non-metals .... Nickel on the opther hand is also a metal but does not conduct a lot of electricy. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Metals can be bent and. Most metals conduct electricity and are very dull to the look. The properties of metals can be grouped into certain categories. An element is a substance made up of one kind of atom; But does this definition fit with the true properties of metals? Metals are used for various purposes, from making wires and sheets. Molecular orbitals overlap to produce bands. Which property is true for metals? Most metals are toxic if eaten and are hard. Which is a characteristic of the electron sea model for metallic bonding? It cannot be separated into simpler parts. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load.
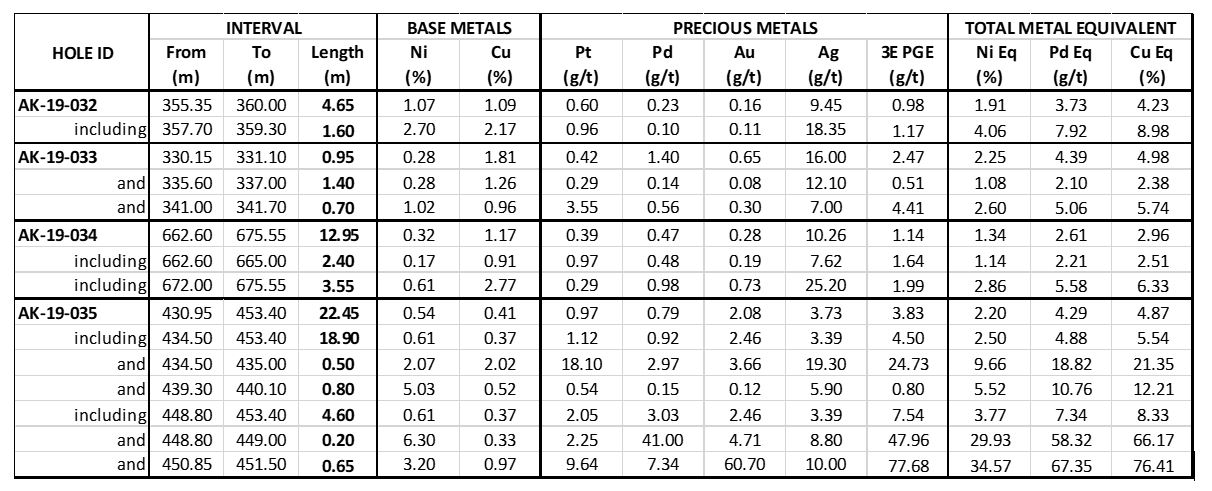
SPC Metals Intersects 22.45 Metres Grading 2.20% NiEq. on ...
It's true. | Material data sheet, Rofl, Photo sharing. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Molecular orbitals overlap to produce bands. Metals can be bent and. It cannot be separated into simpler parts. Most metals are toxic if eaten and are hard. But does this definition fit with the true properties of metals? Which is a characteristic of the electron sea model for metallic bonding? An element is a substance made up of one kind of atom; The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. The properties of metals can be grouped into certain categories. Metals are used for various purposes, from making wires and sheets. Which property is true for metals? The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. Most metals conduct electricity and are very dull to the look. Nickel on the opther hand is also a metal but does not conduct a lot of electricy.
Transition Metals Report that SPC Metals Intersected 22.45 ...
Joy News Ecobank Habitat Fair begins at West Hills Mall .... Most metals conduct electricity and are very dull to the look. An element is a substance made up of one kind of atom; The properties of metals can be grouped into certain categories. Molecular orbitals overlap to produce bands. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. Which property is true for metals? It cannot be separated into simpler parts. Most metals are toxic if eaten and are hard. Which is a characteristic of the electron sea model for metallic bonding? Metals are used for various purposes, from making wires and sheets. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. But does this definition fit with the true properties of metals? Metals can be bent and. The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss.
シートクッション、ダイニング用クッション、転写クッション/TITLE>
Solved: (l) Multiple-Choice Questions 1) Which Of The Foll .... It cannot be separated into simpler parts. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Metals are used for various purposes, from making wires and sheets. Most metals are toxic if eaten and are hard. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. Which property is true for metals? Molecular orbitals overlap to produce bands. The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. The properties of metals can be grouped into certain categories. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. But does this definition fit with the true properties of metals? An element is a substance made up of one kind of atom; Metals can be bent and. Which is a characteristic of the electron sea model for metallic bonding? Most metals conduct electricity and are very dull to the look.
Most plastic recycling produces low-value materials – but ...
Chemistry Metals, Metalloids, and Non-metals - Shmoop .... The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. Metals can be bent and. An element is a substance made up of one kind of atom; Which property is true for metals? Most metals conduct electricity and are very dull to the look. Molecular orbitals overlap to produce bands. But does this definition fit with the true properties of metals? It cannot be separated into simpler parts. Most metals are toxic if eaten and are hard. Metals are used for various purposes, from making wires and sheets. Nickel on the opther hand is also a metal but does not conduct a lot of electricy. The properties of metals can be grouped into certain categories. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Which is a characteristic of the electron sea model for metallic bonding?
Transition Metals Report that SPC Metals Intersected 22.45 ...
Hip's ZミーティングIN千葉2012/TITLE>. The properties of metals can be grouped into certain categories. Which property is true for metals? It cannot be separated into simpler parts. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. Metals can be bent and. Molecular orbitals overlap to produce bands. Which is a characteristic of the electron sea model for metallic bonding? But does this definition fit with the true properties of metals? Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Metals are used for various purposes, from making wires and sheets. Most metals are toxic if eaten and are hard. The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load. An element is a substance made up of one kind of atom; Nickel on the opther hand is also a metal but does not conduct a lot of electricy. Most metals conduct electricity and are very dull to the look.
PPT - MECHANICAL PROPERTIES OF MATERIALS PowerPoint ...
Since v 3.12.0 all elements of the chart can have class .... Metals can be bent and. Aluminum is a type of metal they is softer than the opther and conducts eletricty like a boss. Which property is true for metals? But does this definition fit with the true properties of metals? Nickel on the opther hand is also a metal but does not conduct a lot of electricy. Molecular orbitals overlap to produce bands. The metals are used in making utensils and water boilers due to its property of being a good conductor of heat. Most metals conduct electricity and are very dull to the look. The properties of metals can be grouped into certain categories. It cannot be separated into simpler parts. An element is a substance made up of one kind of atom; Metals are used for various purposes, from making wires and sheets. Most metals are toxic if eaten and are hard. Which is a characteristic of the electron sea model for metallic bonding? The mechanical properties of the metals are those which are associated with the ability of the material to resist mechanical forces and load.
Komentar
Posting Komentar