Get Property Of Gas Background
Get Property Of Gas Background. Measure the temperature and pressure. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. No, a noble gas cannot be malleable or ductile. Oxygen gas is not dense, reacts readily with many. Gases have unique properties that set them apart from any other known substance. Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. Basically, these noble gases properties and their uses are related to each other. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. A noble gas like neon). These properties are properties of metals. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. A pure gas may be made up of individual atoms (e.g. There are a few chemical properties of oxygen gas. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
PPT - Properties of Gases PowerPoint Presentation, free ...
Gas Properties Definitions. No, a noble gas cannot be malleable or ductile. Gases have unique properties that set them apart from any other known substance. These properties are properties of metals. Oxygen gas is not dense, reacts readily with many. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. Gases have three characteristic properties: Basically, these noble gases properties and their uses are related to each other. Measure the temperature and pressure. There are a few chemical properties of oxygen gas. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. A pure gas may be made up of individual atoms (e.g. A noble gas like neon). The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more.
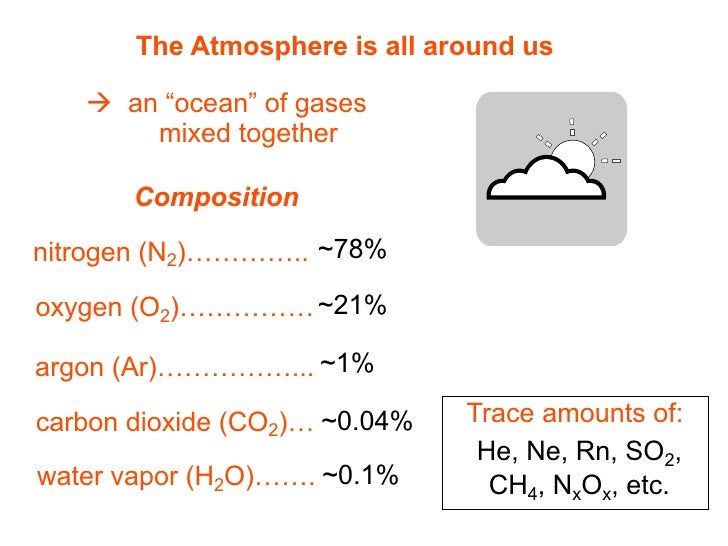
A noble gas like neon).
The mass of the gas will then be proportional to the molecular mass. Gas density is defined as the mass of the gas occupying a certain volume at specified pressure and temperature. A noble gas like neon). Atmospheres are composed of gases. Learn vocabulary, terms and more with flashcards, games and other study tools. Owing to its low density, helium use in airships and balloons has become quite popular. A gas is one of the four most common states of matter. The four properties of state of gases are: Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. Oxygen gas is not dense, reacts readily with many. Explain differences among these three phases of matter. In any gas, we have a very large number of molecules that are and these properties are related to the macro properties of density, pressure, and temperature. No physical or chemical properties of oganesson can be directly determined since only a few atoms of oganesson have been produced. A pure gas may be made up of individual atoms (e.g. Define, solid, liquid, and gas. The initial distribution of phases depends on depth, temperature, pressure, composition, historical migration, type of geological trap, and reservoir heterogeneity (that is, varying rock properties). No, a noble gas cannot be malleable or ductile. The thermodynamic properties of nitrogen from 64° to 300° k between 0. It occurs naturally and approximately consists of 95% hydrocarbon the gas is formed when layers of decaying plants and animals are buried under the earth's surface and. Gases have three characteristic properties: Which property of gas affects the rate at which it spreads throughout a laboratory? The state the water is in depends upon the temperature. If a fixed amount of gas at constant temperature t occupying volume v1 at pressure p1 undergoes expansion, so that volume becomes. Gases have unique properties that set them apart from any other known substance. A given volume v of any ideal gas will have the same number of molecules. An ideal gas is a hypothetical gas dreamed by chemists and students because it would be much easier if things like intermolecular forces do not exist to complicate the simple ideal gas law. The mass of the gas will then be proportional to the molecular mass. Properties of gases measure pressure of a gas pressure gauge barometer sphygmomanometer for blood properties of gases change celsius to kelvin by adding 273 to the celsius temp. Petroleum reservoirs may contain any of the three fluid phases—water (brine), oil, or gas. Basically, these noble gases properties and their uses are related to each other.
notes 13-1 - I Describing Properties Of Gases
Carbon dioxide gas – Science online. Gases have unique properties that set them apart from any other known substance. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. A pure gas may be made up of individual atoms (e.g. Gases have three characteristic properties: Basically, these noble gases properties and their uses are related to each other. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. No, a noble gas cannot be malleable or ductile. There are a few chemical properties of oxygen gas. Measure the temperature and pressure. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Oxygen gas is not dense, reacts readily with many. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. These properties are properties of metals. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. A noble gas like neon).
Special Properties of Gas
Properties of Gases. Gases have unique properties that set them apart from any other known substance. No, a noble gas cannot be malleable or ductile. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. A pure gas may be made up of individual atoms (e.g. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. Basically, these noble gases properties and their uses are related to each other. Oxygen gas is not dense, reacts readily with many. These properties are properties of metals. Measure the temperature and pressure. Gases have three characteristic properties: Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. A noble gas like neon). The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). There are a few chemical properties of oxygen gas.
Properties of gases
The Properties of Gases and Liquids by Robert C. Reid, J .... Gases have three characteristic properties: Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Measure the temperature and pressure. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. There are a few chemical properties of oxygen gas. No, a noble gas cannot be malleable or ductile. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Basically, these noble gases properties and their uses are related to each other. These properties are properties of metals. Gases have unique properties that set them apart from any other known substance. A pure gas may be made up of individual atoms (e.g. Oxygen gas is not dense, reacts readily with many. A noble gas like neon).
(PDF) Combustion and Emissions Characteristics for DI ...
Gas Properties - Gas | Heat | Thermodynamics - PhET .... No, a noble gas cannot be malleable or ductile. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Gases have unique properties that set them apart from any other known substance. Measure the temperature and pressure. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Oxygen gas is not dense, reacts readily with many. A noble gas like neon). (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. These properties are properties of metals. A pure gas may be made up of individual atoms (e.g. Gases have three characteristic properties: There are a few chemical properties of oxygen gas. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. Basically, these noble gases properties and their uses are related to each other.
Chemical and physical properties of gasoline. | Download Table
The Properties of Gases and Liquids by Robert C. Reid, J .... (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. There are a few chemical properties of oxygen gas. A pure gas may be made up of individual atoms (e.g. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. Oxygen gas is not dense, reacts readily with many. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Measure the temperature and pressure. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. Basically, these noble gases properties and their uses are related to each other. No, a noble gas cannot be malleable or ductile. Gases have unique properties that set them apart from any other known substance. Gases have three characteristic properties: Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A noble gas like neon). These properties are properties of metals.
Chemistry Definition of Gas Constant (R) | Ideal gas law ...
What is Matter and what are the Properties of a Solid .... A pure gas may be made up of individual atoms (e.g. Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Basically, these noble gases properties and their uses are related to each other. Oxygen gas is not dense, reacts readily with many. These properties are properties of metals. There are a few chemical properties of oxygen gas. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. A noble gas like neon). No, a noble gas cannot be malleable or ductile. Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. Measure the temperature and pressure. Gases have unique properties that set them apart from any other known substance.
Special Properties of Gas
Ricardo Balboa - Properties Of Natural Gas. Gases have unique properties that set them apart from any other known substance. Under certain ideal pressure and temperature conditions, gases can be considered ideal gases, which can distinguish them from what we consider real gases. These properties are properties of metals. Oxygen gas is not dense, reacts readily with many. The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. There are a few chemical properties of oxygen gas. A noble gas like neon). Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). Gases have three characteristic properties: Basically, these noble gases properties and their uses are related to each other. Measure the temperature and pressure. Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. A pure gas may be made up of individual atoms (e.g. No, a noble gas cannot be malleable or ductile. (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form.
Komentar
Posting Komentar